
Nabilone is a cannabinoid derivative that is structurally different from delta-9-tetrahydrocannabinol (THC), the main psychoactive compound in Cannabis. It is a weak opioid that has therapeutic applications as an adjunct analgesic for neuropathic pain. It is sold under the brand name Cesamet, which is marketed in capsule form. Schedule II of Controlled Substances Act governs this drug. It can pose a danger to users. A review of all published studies confirms that the drug's safety is generally good.
Nabilone was tested for postnatal developmental toxicities in pregnant rabbits and rats. It was also evaluated in a Chinese hamster sister chromatin exchange test, and in the male rat dominant lethal test. These tests showed that it was not toxic. Nabilone was administered at doses between 1 and 12 mg/kg/day in the trial. During the course the study, nabilone's inhibitory effects on CYP2C9 and CYP2A6 were also assessed. There was a moderate inhibition in CYP2C8. In contrast, nabilone did not inhibit CYP2A6.

Although side effects have not been reported to be serious, it is important for patients to be aware of possible side reactions. Some medications may interact with nabilone so be cautious when administering this drug. The drug may cause the displacement of other proteinbound drugs. This is especially true if used with certain medications. Patients who are taking nabilone may be more likely to develop substance abuse disorders. Thus, prescribers and health care professionals should watch for signs of substance use and abuse.
In addition, the drug can cause withdrawal symptoms if used for a long period of time. Do not take it if you have consumed alcohol or other drugs which cause drowsiness. Pregnant women and nursing mothers should not take this medication.
Numerous P450 enzyme isoforms extensively process Nabilone. This means that the drug may accumulate in feces, urine, and blood. Moreover, the metabolites produced by nabilone have peak concentrations comparable to the parent drug. Stereospecific enzyme reduction of the 9-keto moiety in nabilone produces them. These metabolites include carbinol, diol, and nabilone. Depending on the metabolite, the terminal elimination half-life of the drug may be increased.
Patients with psychiatric disorders (e.g., depression or anxiety) should be closely monitored for potential adverse reactions. The drug should be used with caution by patients with heart disease or other cardiovascular conditions. Prescription doctors should limit the amount of medication a patient receives in one cycle of chemotherapy.
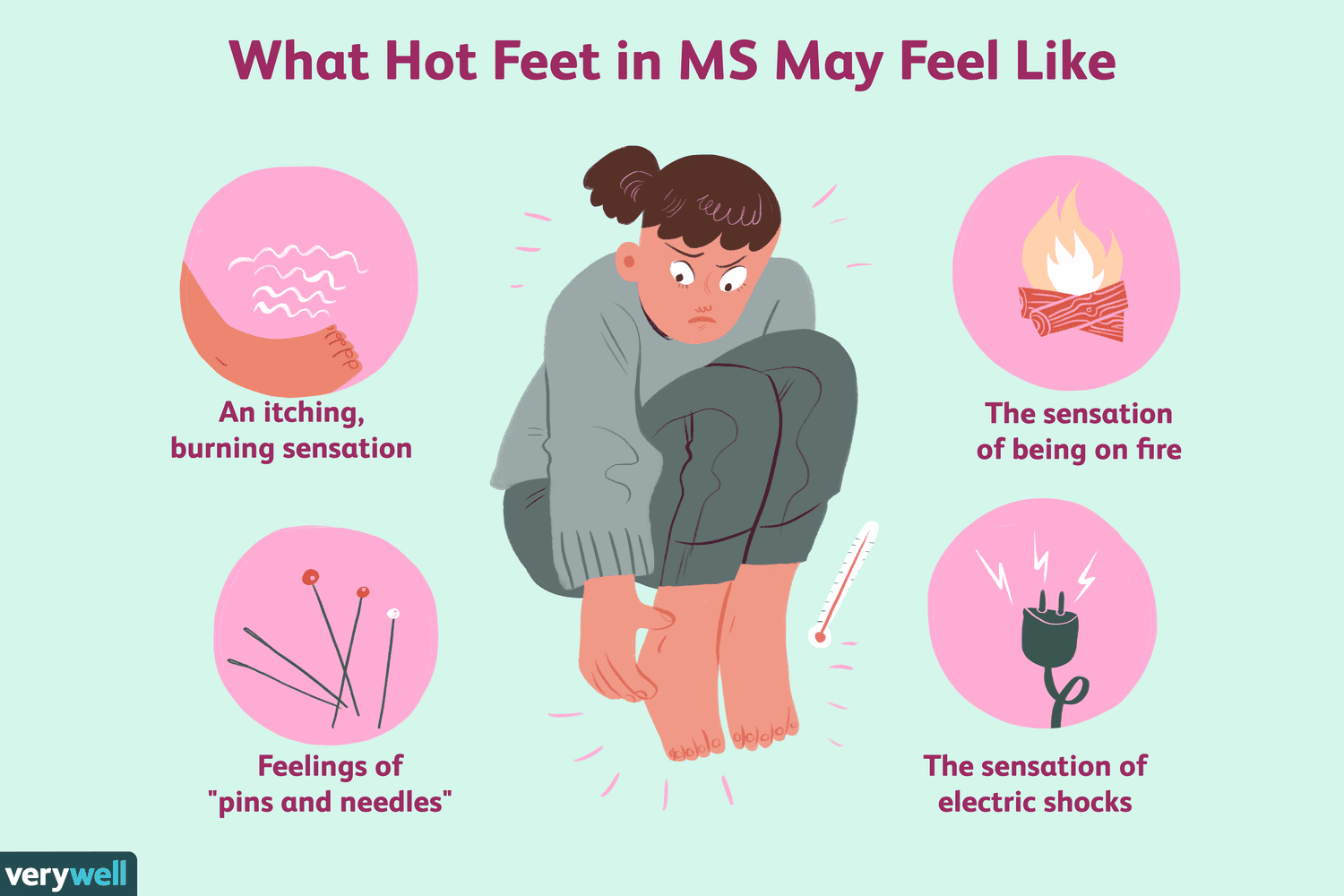
Patients with a history of substance abuse should be closely monitored for signs of abuse. Persons who have a family or personal history of mental illness, such as nabilone abuse, should not take it. Lastly, it should not be administered to children. Children might be more sensitive than adults to the side effects.
FAQ
Is there evidence that CBD reduces anxiety?
CBD oil has the ability to alleviate anxiety by interfacing with certain receptors within the brain, CB1 and CB2. The endocannabinoid process regulates stress responses and mood.
When we feel anxious, our bodies release chemicals that activate the CB1 receptor. This receptor activates and sends signals to amygdala which is responsible for emotional processing.
When the CB1 receptor is blocked, the amygdala doesn't receive the signal to process emotions. CBD users report less negative feelings.
2017 research showed that CBD has been shown to reduce anxiety in those suffering from socialphobia. Another study confirmed that CBD can reduce symptoms associated with PTSD.
A 2018 study concluded that CBD can be used to treat anxiety disorders and anxiolytic effects.
Another study suggested that CBD may also help to reduce panic attacks.
Numerous studies have found that CBD can increase anxiety in mice.
According to the researchers, this discrepancy between animal and human data may be due in part to differences in CBD's effects on humans and animals.
CBD does not have any safety data. Experts agree that CBD is safe when taken as directed.
Is CBD a good idea to invest in?
As hemp-based products gain popularity, so does the market. It's estimated that by 2022 there could be $1 billion worth of hemp-based products on store shelves.
Market growth is expected at an annual rate in excess of 20% up to 2020, when it will reach $2.5Billion.
Hemp oil is used in many beauty- and health-care products like lotions.
Many companies also make CBD-infused snacks, pet food, treats, and other food products.
CBD is legal in all 50 states. However, this could change very soon. CBD will become more widely used as a legal substance in the future. This will allow businesses to be more legally able to do business.
With these factors in mind, it's clear that investing in CBD can be a lucrative venture.
What are the best CBD brands available?
These top CBD brands have been hand-picked by us based on their quality, reliability, value, and other criteria.
They sell high-quality CBD oil products with less than 0.2% THC.
We also recommend checking out our list of the best CBD sellers worldwide.
Which CBD products are most popular?
CBD products are becoming increasingly popular. They can be used for anything, including pain relief or anxiety. The market is big and growing fast.
But for what purpose do people buy CBD? And how does this affect you as a brand owner?
Statista says CBD products are popular for their relaxing properties. They are also purchased for their antiinflammatory properties.
This means that if your product has both CBD and THC, then it can be sold for both recreational and medicinal purposes.
But what about brands which are focused on just one purpose? A company selling CBD for stress relief is an example of a brand that will not be challenged.
Also, if a brand is focused on CBD for medical reasons, it will have large customers.
However, if a brand wants to target recreational users, then they need to create a unique selling proposition (USP). A USP simply means a distinctive feature or benefit that differentiates a brand's competitors.
For example, some brands offer free shipping, while others offer discounts for bulk orders.
Can I use CBD during pregnancy?
There isn't enough research to know if CBD is safe to use during pregnancy.
But based on the limited amount of information available, it appears unlikely that CBD would cause harm to the baby.
It's important to note that CBD should not be taken by pregnant women unless recommended by their doctor.
A recent warning was issued by the Food and Drug Administration about possible risks from CBD consumption during pregnancy.
FDA stated that there was evidence that marijuana use during pregnancy might increase the risk for miscarriage.
The agency stated that further research is required before a firm conclusion could be drawn.
Is the CBD market saturated or not?
CBD industry is growing at over 25% per annum. This growth rate is expected to continue at least for five more years. The industry is forecast to grow from $2 Billion to $5 Billion by 2020.
Two companies dominate the CBD market: GW Pharmaceuticals (Canndoc Ltd) and Canndoc Ltd. Both companies are focused on the development of pharmaceutical-grade CBD products. They haven't been very successful so far. Both are struggling to gain traction on the market.
Cannabidiol or CBD is a form of cannabis extract with less than 0.3% HHC. It doesn't produce any psychoactive effects. It is used as a treatment for epilepsy and other medical conditions. It is also used frequently as a dietary addition.
There are many varieties of CBD products. Some CBD products are made with whole plants extracts, others use CBD isolates.
All of these products share one thing: They contain low levels THC.
They are therefore legal under US federal law. You still need to comply with local laws when you sell CBD products. Always check your state's laws regarding CBD products.
Additionally, CBD products in some states are illegal. These include California, Colorado, Florida, Mississippi, Missouri, New York, North Carolina, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Dakota, Texas, Utah, Virginia, Washington, and Wisconsin.
CBD products shouldn't be used if you live in any of these states.
How can CBD products be promoted in a legal manner by CBD companies?
The FDA does no regulate hemp as a crop commodity. The Controlled Substances Act regulates all cannabis derivatives, including marijuana. To date, there are no specific regulations for CBD.
CBD is legal at state level in 29 US states. Federal law considers it illegal. This uncertainty creates uncertainty for CBD product sellers.
The FDA also maintains strict guidelines on how CBD products may be marketed. To make sure that CBD products are clearly disclosed about their THC content, the FDA has established strict guidelines. Without scientific evidence supporting this claim, CBD cannot be used to treat certain medical conditions.
Additionally, the FDA requires manufacturers submit information about manufacturing practices and quality control. They require companies to carry out clinical trials to prove safety or efficacy.
These are important considerations for companies when creating their marketing strategies.
Statistics
- While the primary injury may not be treatable, interventions that attenuate secondary sequelae are likely to be of benefit [203].Only one study (ncbi.nlm.nih.gov)
- The inhibition of FAAH is predicted to lead to an increase in brain and plasma concentrations of AEA, which acts as a partial agonist at CB1R and CB2R, thereby increasing endocannabinoid tone [92, 110]. (ncbi.nlm.nih.gov)
- A recent systematic review of human trials also reported that individuals with epilepsy receiving CBD (5–20 mg·kg−1·day−1) were more likely to experience decreased appetite than those receiving placebo (i.e., ~20 vs. 5% of patients) (ncbi.nlm.nih.gov)
- The use of these products is likely to become even more widespread if the World Health Organization's recommendation that CBD no longer is scheduled in the international drug control conventions is adopted by the United Nations member states [201]. (ncbi.nlm.nih.gov)
- As a substance that was federally illegal before the passage of the 2018 Farm Bill, hemp-derived cannabinoids with no more than 0.3% THC still face a regulatory grey area. (forbes.com)
External Links
How To
How to get certified for selling CBD products
CBD (cannabidiol) is one of the hundreds of cannabinoids found in cannabis plants. It has been used medicinally throughout history. This includes in South America, China, India and China. Because it can treat conditions such as anxiety, pain, epilepsy and inflammation, CBD has seen a rise in popularity over the years. But if you want to start selling CBD products, there's no official certification program available yet -- at least not in the U.S. That means anyone who wants to make money off their own line of CBD products has to rely on the "unofficial" process of self-certification.
There are two options. One way to do this is to join the local association of cannabis-business owners. This will allow you to share your knowledge with others, as well as receive advice and support. There are currently many associations across the country. There are two options. The first is to open an online business. Many states allow canna businesses to operate online. If so, then you can set up your own website and begin taking orders right away. However, you will still need to register at your state's Department of Public Health. Once you have registered, it will be possible to apply for your license through the state's department public health. After receiving your license, you are legally allowed to open a store and start accepting orders.